Gastrointestinal stromal tumors (GIST): retrospective study of 6 dogs
Tumores estromales gastrointestinales (GIST): estudio retrospectivo de 6 perros
I. Montañés, A. Vila, X. Roura, L. Santos, A. Canturri, J. Verdés, L. Martín, C. Díaz-Bertrana, A. Lloret
Contacto: ivanmontanessancho@gmail.com
Resumen
Los tumores de estroma gastrointestinal (GIST, por sus siglas en inglés) son un grupo de neoplasias mesenquimales caracterizadas por la expresión de la proteína c-kit (antígeno CD117). El objetivo de este estudio es caracterizar los hallazgos clínicos y anatomopatológicos asociados con GIST en perros visitados en nuestro hospital entre 2000 y 2018. Seis perros fueron retrospectivamente seleccionados y estudiados. Los GIST se diagnosticaron en perros mayores, 3 de los 6 perros presentaban signos inespecíficos y los otros 3 no mostraban signos clínicos. Todos los tumores fueron positivos frente a inmunohistoquímica para CD117. En 5 de los 6 perros los tumores se localizaron en el ciego. Solamente un perro tuvo una recaída y presentó una hipoglicemia supuestamente secundaria al GIST. Este síndrome paraneoplásico es muy raro y ha sido escasamente descrito en perros. Los inhibidores de los receptores tirosin-kinasa han sido efectivos en el tratamiento de estos tumores en perros, aunque actualmente no hay disponibles protocolos de tratamiento estandarizados. Los resultados de nuestro estudio son similares a otros publicados. Los GIST representan una entidad clínica y anatomopatológica específica, que se localizan principalmente en el ciego y muestran un amplio espectro clínico, desde tumores indolentes a casos con metástasis.
Palabras clave: intestino, proteína c-kit, inhibidores de los receptores tirosin-kinasa, hipoglicemia, perro.
Clin Vet Peq Anim 2019, 39 (3): 155-161
Summary
Gastrointestinal stromal tumors (GIST) are a specific group of mesenchymal tumors characterized and distinguishable from other mesenchymal tumors by the expression of c-kit (CD117 antigen). The objective of this study is to characterize clinical and pathological data associated with canine GIST presented at our hospital between 2000 and 2018. Six cases were retrospectively enrolled and clinical data reviewed. This kind of tumors occurred in old dogs, clinical signs were no specific and up to 50% of dogs were asymptomatic at presentation. All tumor samples submitted after surgery yielded positive results on immunohistochemistry for CD117. Five out of 6 tumors were located at the cecum. Only one dog had a recurrence showing hypoglycemia presumptively associated with GIST, which is a rare paraneoplastic syndrome poorly described in dogs. Tyrosine kinase inhibitors have been effective in the treatment of canine GIST, although no standardized protocols are currently available. In conclusion, our findings were similar to previously reported ones in which GIST have been defined as a specific clinical and pathological entity, located mainly in the cecum and with a broad clinical spectrum from indolent cases to those with metastasis.
Keywords: bowel, c-kit, tyrosine kinase inhibitors, hypoglycemia, canine.
Clin Vet Peq Anim 2019, 39 (3): 155-161

La presencia de este logo en un artículo de la revista indica que se publicará un examen sobre el mismo en la plataforma AVEPA Elearning. Su resolución aporta 0,15 créditos dentro del sistema de acreditaciones de especialidades veterinarias de AVEPA.
Introduction
Primary intestinal tumors account for less than 10% of all neoplasms in dogs and may be of epithelial, neuroendocrine, hematopoietic, or mesenchymal origin.1 Gastrointestinal stromal tumors (GIST) are a specific group of mesenchymal neoplasms that have been reported in humans, dogs, and other species.2-5 Historically, these tumors were misclassified as leiomyosarcomas or leiomyomas in dogs due to their similar histological features, but in recent studies 30-70% of gastrointestinal mesenchymal tumors were reclassified as GIST based on immunohistochemical staining (IHC).2-4 They are pleomorphic tumors characterized by the expression of c-kit protein (CD117 antigen) which is a receptor tyrosine kinase encoded by the c-kit proto-oncogene.2-5 These tumors are thought to arise from interstitial cells of Cajal (ICC) which are intestinal pacemaker cells that on light microscope have characteristics of both smooth muscle and neural differentiation. Neoplastic ICC can preferentially express one, both, or neither of these features, thus accounting for the variants of GIST: smooth muscle, neural, mixed or anaplastic.2,5,6 In humans, the development of GIST is largely driven by gain-of-function mutations in the c-kit gene or less commonly the platelet-derived growth factor receptor-alpha (PDGFRA) gene.6,7 Several and similar activating mutations of the c-kit proto-oncogene, mainly located on exon 11, have been identified in human and dogs with these kind of tumors.3,7 Therefore, it is thought that the pathogenesis is very similar in both species, albeit no mutations on PDGFRA gene have been identified in dogs.1,7
These neoplasms can arise from the omentum, mesentery, retroperitoneum and anywhere in the gastrointestinal (GI) tract.1,5,6 In dogs most GIST arise from large intestine, mainly from the cecum,2-4 whereas in humans are mostly located in stomach and small intestine.6,8 These tumors have a very broad clinical behavior from asymptomatic cases to those that occur with metastasis, being the liver the most common location for metastasis.1,6,9 The c-kit expression is the hallmark of these tumors and the gold standard of its diagnosis in dogs and humans.1,4,7 However, studies estimate that up to 15% of human GIST stain negative or only weakly positive for c-kit protein.10 These tumors, named wild-type GIST,7 are clinicopathologically indistinct from c-kit-positive GIST.7,10 Studies in humans have identified DOG1 (discovered on gastrointestinal stromal tumors protein 1) as a highly sensitive and specific marker for human GIST.7,10 A recent report suggested that a combined immunohistochemical staining assessment for c-kit and DOG1 is most sensitive for diagnosis of these tumors in dogs.11 Complete resection, together with tumor-free margins and avoidance of tumor rupture, remain the best option for a curative approach for resectable canine and human tumors.1,2,6,12 However, above 50% of human patients ultimately experience recurrence or metastasis after surgical resection and about one half of GIST are overtly metastatic at presentation.5,8,12 Tumor sizes, mitotic index, presence of rupture and tumor site are considered as the most important parameters for human patients to predict clinical behavior after surgery.6,8,12 No conclusive data are currently available to predict the clinical behavior of GIST in dogs after surgery.1,2,9,13 In human medicine adjunctive therapy based on tyrosine kinase inhibitors (TKI) is considered the standard of care for patients with high risk for metastasis after surgery, presented with metastasis and for non-resectable or recurring tumors.6,8,12 In a recent case report of one dog with non-resectable tumor receiving imatinib (Glivec®, Novartis Pharma AG, Basel, Switzerland) a partial remission for 140 days was achieved.14 In other case report one dog with recurrent metastatic GIST had a complete remission after two months of treatment with imatinib. This dog died 4 years later due to pneumonia with no evidence of recurrence.15 Toceranib phosphate (Palladia®; Zoetis, Parsippany, New Jersey) was successful for disease control over 9 months in a dog with metastatic GIST.16 A retrospective study of 27 canine GIST showed evident clinical benefits of toceranib.17 Therefore, TKI have shown clinical benefit in cases of canine GIST as well, although no standard protocols have been published.
The aims of this study were to describe the prevalence, clinical and pathological features associated with GIST in dogs at our referral hospital and to compare these data with previously reported to establish a standardized clinical approach for future canine GIST cases.
Material and Methods
A retrospective search of canine GIST was performed in the files of the pathology service of the veterinary school since 2000 to 2018. The keywords used were “mesenchymal tumors”, “c-kit” and “gastrointestinal stromal tumors”. Inclusion criteria were dogs with a diagnosis of GIST based on histological features and immunohistochemistry (c-kit positive). For each dog the following data were reviewed and recorded: signalment, clinical signs at presentation, blood test results, imaging findings, surgical findings, histological findings, postsurgical treatment and follow-up information after surgery and survival time. The information was obtained from internal medicine service records and when necessary from the owners and referring veterinarians through email or phone calls. The lack of some information was not an exclusion criterion.
Results
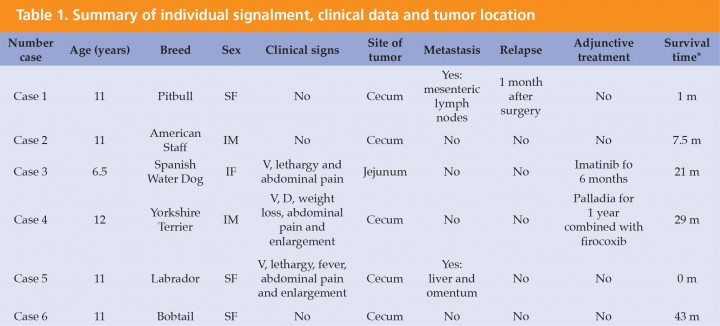
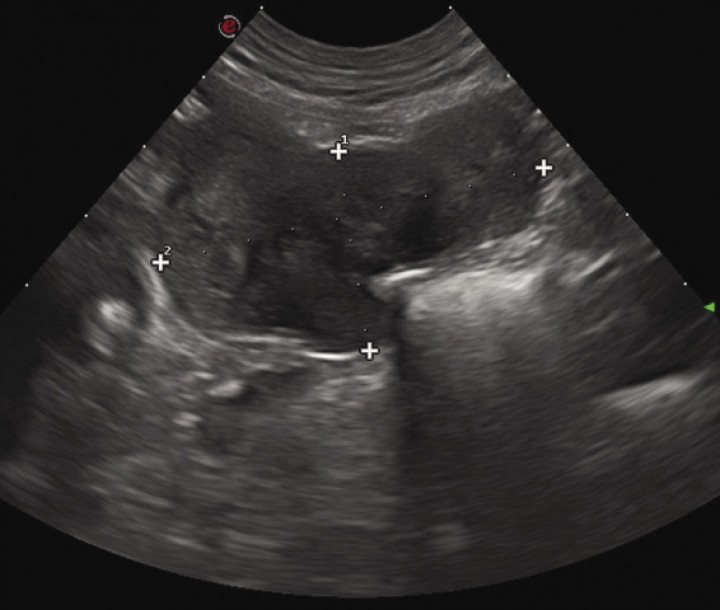
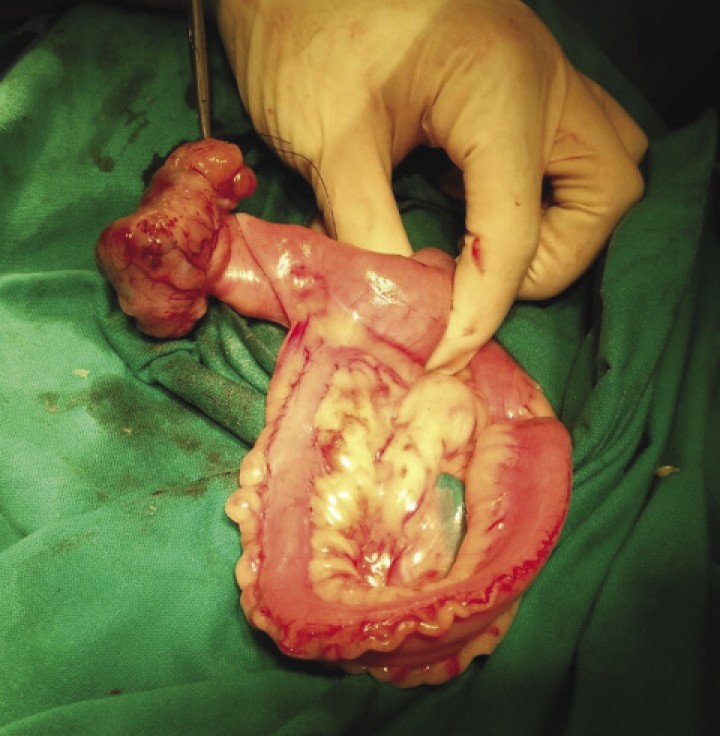
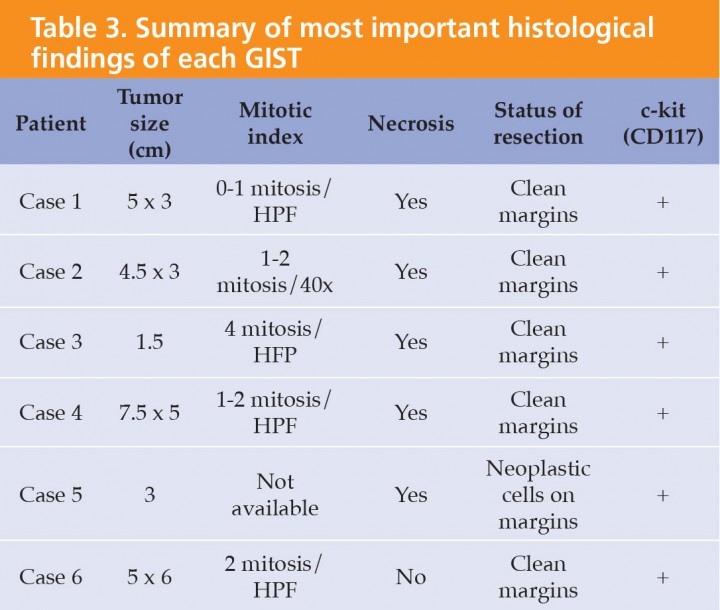
Six dogs with a histological confirmed diagnosis of GIST and c-kit positive results were enrolled in the study. One dog previously diagnosed of leiomyoma was diagnosed with GIST at the beginning of the study after immunohistochemical assessment. There were 4 females and 2 males of the following breeds: Bobtail, American Pitbull, American Staffordshire Terrier, Spanish Water Dog, Labrador, and Yorkshire Terrier. Mean age at presentation was 10.8 years ranging from 6.5 to 12.1 years. Clinical signs at presentation were nonspecific in 3 dogs including anorexia (3), lethargy (3), vomiting (3), abdominal pain and enlargement (3), diarrhea (1), weight loss (1) and fever (1). Three dogs had no any clinical sign related to the GIST (Table 1). Blood tests did not show any relevant abnormalities. In all 6 dogs ultrasonography revealed the presence of abdominal masses (Fig. 1) suggesting a diagnosis of neoplastic disease. A clear link between abdominal mass and specific abdominal structure was undetermined in 3 out of 6 cases, although in one dog the mass was thought to be in contact with intestinal loops (Table 2). Thoracic radiographs performed in 4 out of 6 dogs did not show any evidence of metastasis. All dogs underwent laparotomy and two dogs were previously diagnosed with abdominal perforation, which was confirmed at surgery. One of these two dogs was euthanatized during surgery because of the presence of gross metastasis in the liver and omentum. These findings were missed on the abdominal ultrasound and were subsequently confirmed at necropsy. One of the GIST was located on jejunum, and the other 5 on cecum (Fig. 2). In all 5 dogs, complete macroscopic resection was achieved. Peri-operative complications were not seen in any case except for one dog that developed acute pancreatitis, but he had a good outcome being discharged 6 days later. Immunohistochemical assessment yielded generalized positive results for c-kit in all samples, thus confirming the diagnosis of GIST (Fig. 3). Only one out of 6 tumors was incomplete resected based on light microscope. Specific histological details are summarized in Table 3. Adjunctive therapy with TKI after surgery was recommended in 3 cases and was declined in one case. One dog underwent treatment course of imatinib mesylate (Glivec®, Novartis Pharma AG, Basilea) at a dose of 12 mg/kg/PO/SID for 6 months and not adverse effects related to treatment were seen. The remaining dog received a combination of firocoxib (Previcox®, Merial Laboratorios, S.A., San Cugat del Vallés) and toceranib (Palladia®, Zoetis, Parsippany, New Jersey) at a dose of 2.5 mg/kg/PO three times per week for 1 year. The treatment was discontinued due to side effects including vomiting and diarrhea. One dog had a relapse 36 days after surgery. Blood test at that moment revealed hypoglycemia and abdominal ultrasound findings suggested metastatic process located on mesenteric lymph nodes although it was not histologically confirmed. Initially hypoglycemia was controlled with a continuous 5% dextrose infusion and treatment with TIK was proposed as long-term therapy, but owners declined further treatment. Mean survival time for the 6 dogs (including the one euthanized at surgery) was 17.5 months. Mean survival time for the 5 dogs was 20.6 months (range from 1 to 43 months). Two of them were still alive by the time of writing this paper and did not show any evidence of relapse.
SF: spayed female, IM: intact male, IF: intact female, V: vomiting, D: diarrhea.
*after surgery
Figura 1
Ultrasound appearance of one of the GIST located on cecum.
LN: lymph nodes.
Figura 2
Macroscopic aspect of the GIST showed in Figure 1.
Figura 3
Immunohistochemistry c-kit. (A) Histophatological appearance of one GIST. It shows marked and diffuse positive immunohistochemical staining for c-kit (CD117), x40. (B) Closed image x400.
Discussion
The true incidence of canine GIST has not been established1 but the occurrence at our hospital seems to be lower compared to other studies.2-4,9 One dog diagnosed with leiomyoma was reclassified with GIST during the study. Therefore, the prevalence of these tumors at our hospital could be underestimated as reported in earlier studies.2-4 The signalment and clinical sings at presentation were consistent with previous reports.2-4,9,18 Subclinical cases of GIST are relatively frequent in humans8 and up to 75% of tumors were an incidental finding at necropsy in one study in dogs.3 Similarly, in our dogs 50% of tumors were an incidental finding.
In our study abdominal ultrasound was 100% sensitive for diagnosis of abdominal tumor disease, but failed to both determine the anatomical origin of the tumor in three cases and to detect liver and omental metastasis in one case. Nevertheless, in dogs abdominal ultrasound has been reported as very accurate test to identified the actual tumor origin and to predict metastasis.9,18 In human medicine, ultrasonography is the first line diagnostic test in cases of GIST but in some cases, such as large abdominal masses without clear anatomic, computed tomography (CT) is the best option.6,8 Therefore, in dogs with large abdominal masses CT should be considered prior to surgery. One study in dogs revealed that the presence of an irregular margin pattern in ultrasound could be a marker for malignant behavior,9 similar to humans.20 The same authors suggested that internal echogenicity could be a prognostic marker for metastasis as tumors with large internal hypoechogenic areas (indicative of necrosis areas) were categorized as high-risk. Moreover, these tumors tended to be larger in size than types categorized as intermediate or low-risk.9 These results agreed with earlier studies reporting that areas of necrosis positively correlated with tumor size13 and larger tumor diameter could be a negative prognostic indicator in dogs.2 However, these studies had some limitations, so these conclusions should be assumed with caution. Similar to other studies2,4,18 thoracic metastasis were not seen in our patients.
In our study 5 of 6 tumors were localized on the cecum, similar to previous reports.2,4,9 Complete resection was achieved in 5 out of 5 dogs with primary localized disease and recurrence rate after surgery was 20% which is similar to some reports2,3 and higher4 than in others. In humans, selection of the patient for adjunctive treatment after surgery is based on a risk scheme.12 In dogs no conclusive parameters have been reported to predict the clinical behaviour of GIST after surgery, except for some potential markers.2,9,13,17 However, due to the high rate of local and distant recurrence in humans (35% to 60%)5,8,19 we proposed to treat 3 out of 4 dogs which were suitable for adjunctive therapy at that moment. In humans the effectiveness of the treatment depends on the presence and locations of both c-kit and PDGFRA mutations, therefore mutational analysis is paramount in clinical decision-making in all cases.8,12 However, treatment with TKI, is recommended in all human cases expressing c-kit other than tumors containing a PDGFRA D842V mutation.10,12 It is supposed that mutational status play a similar role in dogs than in humans7 but TKI has been effective in both dogs with identified c-kit mutations14,15 and without c-kit mutations.17 In our study all tumors yield positive immunohistochemical results for c-kit, thus all were potential candidates for treatment despite the unknown mutational status. The optimal treatment duration of adjunctive therapy is not still known neither in humans12 nor in dogs.14-17 Some authors have proposed long-term treatment in human patients with advanced disease unless adverse effects are evident.21 However, other studies have suggested that long-term treatment may lead to the emergence of drug-resistant clones.22 In our study reasons to discontinue the treatment were due to owner decision and side effects related to treatment. The mean survival time after surgery was 20.4 months which is lower than previously reported,2,4 although two dogs were still alive by the time of writing the study.
In this study only one dog had a relapse showing weakness and lethargy 36 days after surgery. Blood test did not display abnormalities other than hypoglycemia. Abdominal ultrasound findings were consistent with mesenteric lymph nodes metastasis although these findings were not confirmed by histopathology. In human medicine hypoglycemia in the setting of decrease insulin levels is suggestive of a diagnosis of non-islet cell tumor hypoglycemia (NICTH).22 This paraneoplastic syndrome is very rare in humans and secondary to increased production of an altered form of insulin-like growth factor (IGF-2), named “big” IGF-2.23-26 In our patient neither the insulin levels nor IGF-2 levels were measured, but given the clinical history, biochemical and abdominal ultrasound findings, other causes of hypoglycemia were ruled out and hypoglycemia associated with metastatic GIST was suspected. Early diagnosis and complete tumor resection are the treatment of choice when a NICTH is diagnosed. While waiting for surgical resection IV glucose or dextrose could be used, but often is not enough to prevent hypoglycemia. In such cases, several local antitumor therapies for disease control are suggested. Continuous glucagon infusion can be successful as well. Glucocorticoids have demonstrated to be very effective as single therapy or in combination with other therapies.23-26 In our case hypoglycemia was initially well controlled by means of the administration of continuous 5% dextrose infusion over 24h but surgical resection was not an option because of disseminated metastatic presentation. In humans TKI have been used successfully for long-term control of hypoglycemia associated with unresectable or metastatic GIST.25,26 As TKI has been effective for control of metastatic GIST in dogs,14,15 a treatment with imatinib was proposed for this dog but finally owners elected the euthanasia. To our knowledge, in the veterinary literature there are only three possible cases of dogs with hypoglycemia associated to GIST. These three dogs had a primary, localized disease and hypoglycemia was resolved after surgical removal of the tumor. Insulin levels and IGF-2 were not measured. Therefore, to our knowledge this is the first report of a dog with a presumptive diagnosis of hypoglycemia associated with metastatic GIST. The main limitations of this study are the descriptive nature of the study, retrospective analyses of information and the small number of dogs.
In conclusion, our findings in canine GIST are similar to other papers in which dogs exhibited a broad clinical behavior from indolent tumors to metastatic disease2,4,5,11,17 and paraneoplasic syndromes.2 The finding of cecal tumor on ultrasonography should rise high suspicion for diagnosis of GIST, as cecum is the most common location of these tumors.2-4,11 The prevalence of GIST could be underestimated, so that IHQ stains should be always performed if there is a clinical suspicion of GIST, as c-kit expression is the cornerstone for the diagnosis of these tumors. When possible, complete surgical resection is the treatment of choice but for unresectable or metastatic tumors TKI have demonstrated to be effective.1 ,14-17 Due to the lack of standard treatment protocols, we recommend to base clinical decisions on published studies,10,14-17 clinical experience, drug toxicity and suggested prognostic factors.2,11,13,17 Similarly to human consensus,6,12 we recommend performing clinical controls based on CBC, biochemistry and abdominal ultrasound 1 month after surgery and then every 3-6 months for at least 2 years. More studies are needed in order to characterize the clinical behavior of canine GIST and to establish standardized treatment protocols.
Acknowledgements
The authors are grateful to staff members from the Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona (UAB) and Servei de Diagnòstic de Patologia Veterinaria (SDPV) de la UAB who collaborated to manage the cases included in this study. The authors are grateful to owners that allow including their dogs in the study.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article.
Bibliografía
- 1.
Liptak JM and Withrow SJ: Cancer of the Gastrointestinal Trac. In Duncan L (ed): Withrow and Macewen´s Small Animal Clinical Oncology, Missouri, Saunders Elsevier, 2007; 455-510.
- 2.
Maas CPHJ, Haar GT, Van Der, Gaag I, Kirpensteijin, J: Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histological, and immunohistochemical evaluation. Vet Surg 2007; 36:302-313.
[PubMed] - 3.
Frost D, Lasota J, Miettinen M: Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol 2003; 40:42-54.
[PubMed] - 4.
Russell K, Mehler S, Skorupski K, et al: Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990–2003). J Am VetMed Assoc 2007; 230(9):1329-1333.
[PubMed] - 5.
DeMatteo RP, Lewis JJ, Leung D, et al: Two hundred gastrointestinalstromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231: 51-58.
[PubMed] - 6.
Corless CL: Gastrointestinal stromal tumors: what do we know now?. Modern Pathology 2014; 27:1-16.
[PubMed] - 7.
Gregory-Bryson E, Bartlett E, Kiupel M, Hayes S, Yuzbasiyan-Gurkan V. Canine and human gastrointestinal stromal tumors display similar mutations in c-KIT exon 11. BMC Cance. 2010; 10:559.
[PubMed] - 8.
Peixoto A, Costa-Moreira P, Silva M, et al: Gastrointestinal stromal tumors in the imatinib era: 15 years’ experience of a tertiary center. J Gastrointest Oncol 2018 9(2):358-362.
[PubMed] - 9.
Hanazono K, Fukumoto S, Hirayama K, et al: Predicting metastatic potential of gastrointestinal stromal tumors in dog by ultrasonography. J Vet Med Sci 2012; 74:1477-82.
[PubMed] - 10.
West RB, Corless CL, Chen X, et al: The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of c-kit or PDGFRA mutation status. Am J Pathol 2004; 165:107-113.
[PubMed] - 11.
Dailey DD, Ehrhart E, Duval DL, et al: DOG1 is a sensitive and specific immunohistochemical marker for diagnosis of canine gastrointestinal stromal tumors. J Vet Diagn Invest 2015; 27:268-277.
[PubMed] - 12.
Reichardt P, Blay JY, Boukovinas I, et al: Adjuvant therapy in primary GIST: state-of-the-art. Annals of Oncology 2012; 23:2776-2781.
[PubMed] - 13.
Gillespie, V., Baer, K., Farrelly, J., Craft, D. and Luong, R: Canine gastrointestinal stromal tumors: Immunohistochemical Expression of CD34 and examination of prognostic indicators including proliferation makers Ki67 and AgNOR. Vet Pathol 2011: 48: 283-291.
[PubMed] - 14.
Kobayashi M, Kuroki S, Ito K, et al. Imatinib-associated tumour response in a dog with a non-resectable gastrointestinal stromal tumour harbouring a c-kit exon 11 deletion mutation. Vet J 2013 198:271-4.
[PubMed] - 15.
Irie M, Ohtake Y, et al. Imatinib mesylate treatment in a dog with gastrointestinal stromal tumors with a c-kit mutation. J Vet Med Sc 2015; 77(11):1535-9.
[PubMed] - 16.
Elliot JW, Swinbourne F, Parry A, Baines L. Successful treatment of a metastatic, gastrointestinal stromal tumour in a dog with Toceranib phosphate (Palladia). J Small Anim Pract 2017; 58:416-418.
[PubMed] - 17.
Berger EP, Johannes CM, Jergenes AE, et al. Retrospective evaluation of toceranib phosphate (Palladia) use in the treatment of gastrointestinal stromal tumors of dogs. J Vet Intern Med 2018; 32:2045-2053.
[PubMed] - 18.
Hobbs J, Sutherland-Smith J, Penninck D, et al: Ultrasonographic features of canine gastrointestinal stromal tumors compared to other gastroinetestinal spindle cell tumors. Vet Radiol Ultrasound 2015 56(4):432-8.
[PubMed] - 19.
Gold JS, DeMatteo RP: Combined surgical an molecular therapy: the gastrointestinal stromal tumor model. Ann Surg 2006; 244:176-184.
[PubMed] - 20.
Ohashi S, Okamura S, Urano F, et al: Clinical malignancy risk of assessed by endoscopic ultrasonography. Dig Endosc 2006 18:256-262.
- 21.
Le Cesne A, Ray-Coquard I, Bui BN, et al: Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol 2010; 11:942-9.
[PubMed] - 22.
Corless CL, Barnett CM, Heinrich MC: Gastrointestinal stromal tumours: origin and molecular oncology. Nature Reviews Cancer 2011; 11:865-878.
[PubMed] - 23.
Bodnar TW, Acevedo MJ, Pietropaolo M: Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab 2014 99:713-2.
[PubMed] - 24.
Guiteau J, Fanucchi M, Folpe A, Staley CA 3rd, Kooby DA: Hypoglycemia in the setting of advanced gastrointestinal stromal tumor. Am Surg 2006; 72:1225-30.
[PubMed] - 25.
Wilson JM, Ginsberg J, Cutts K, Urban S: A case of Non-Islet Cell Tumor ypogycemia (NICTH) associated with gastrointestinal stromal tumor (GIST). Am J Case Rep 2017 18:984-988.
[PubMed] - 26. [PubMed]
Saeed Z, Taleb S, Evans-Molina C: A case of extragastrointestinal tumor complicated by severe hypoglycemia: a unique presentation of a rare tumor. BMC Cancer 2016; 16:930.