Panniculitis and concurrent inflammatory bowel disease in a dog
Paniculitis y enfermedad inflamatoria intestinal concurrentes en un perro
R.M. Almela, F. Martínez-Gomariz, J. Hervás-Rodríguez, V. Vicente-Calderón, M. Carrión-Jiménez
Contacto: rmalmela@icloud.com
Resumen
La paniculitis es una inflamación de causa multifactorial del tejido adiposo subcutáneo. En medicina humana el erythema nodosum (EN) es la variante más frecuente de paniculitis y ha sido asociado a enfermedad inflamatoria intestinal (IBD). Sin embargo, esta asociación no ha sido todavía confirmada en la literatura en el perro. Este caso clínico describe los hallazgos clínicos e histopatológicos, el examen endoscópico, el tratamiento y el seguimiento de 12 meses en un perro diagnosticado de paniculitis de causa desconocida y aparición de IBD concurrente tras una exacerbación de las lesiones cutáneas. En medicina humana se ha descrito la asociación entre IBD y paniculitis, con posterior resolución de los nódulos tras el tratamiento de la IBD.
Palabras clave: perro, enfermedad inflamatoria intestinal, paniculitis nodular estéril.
Clin. Vet. Peq. Anim, 2018, 38 (3): 171 -174
Summary
Panniculitis is a multifactorial inflammatory condition of the subcutaneous fat. In humans, erythema nodosum (EN), the most frequent variant of panniculitis has been reported in association with inflammatory bowel disease (IBD). However, panniculitis and concurrent IBD have not yet been confirmed in the dog. This case report describes the clinical and histopathological findings, endoscopic examination, treatment, and 12 months outcome in a dog with panniculitis of unknown etiology and development of concurrent IBD after exacerbation of the cutaneous signs. Panniculitis-associated IBD and resolution of skin nodules after treatment of IBD have been reported in humans.
Keywords: dog, inflammatory bowel disease, sterile nodular panniculitis.
Clin. Vet. Peq. Anim, 2018, 38 (3): 171 -174
Introduction
Panniculitis refers to a multifactorial inflammatory condition of the subcutaneous fat.1,2 In human medicine, erythema nodosum (EN) is the most common form of panniculitis and may be associated with a wide variety of diseases including infections, drugs, neoplasia and miscellaneous conditions such as IBD.3 In human and veterinary medicine the diagnosis of IBD is confirmed after clinical, endoscopic and histopathological evaluations.4,5 In humans, dogs and cats, multiple etiologic factors are involved in the genesis of panniculitis including infectious and noninfectious etiologies.1,2,6 This report describes the clinical and histopathological findings, endoscopic examination, treatment, and 12-month outcome in a dog diagnosed with panniculitis, with no underlying cause identified, and concurrent IBD, confirmed after endoscopic and histological examinations.
Case history
An 8-year-old neutered male Dachshund was referred for evaluation of 7-months history of recurrent multifocal subcutaneous nodules (Fig. 1) with hyporexia, weight loss, tenesmus, diarrhea and vomiting after a flare of the skin nodules. 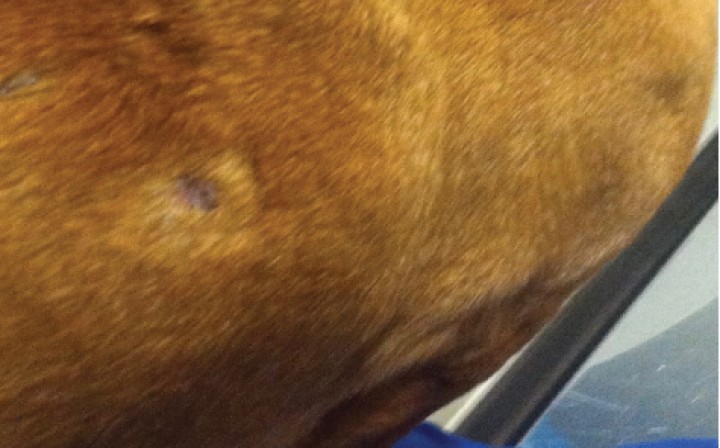
Figura 1
Clinical findings in a dog with panniculitis and concurrent inflammatory bowel disease. Appearance of the subcutaneous partly alopecic nodules on the dorsolateral neck and trunk.
Full-thickness excisional skin biopsies were taken. Aseptically obtained deep tissue specimens were submitted for aerobic and anaerobic, mycobacterial, and fungal cultures. All cultures were negative. Histopathologic examination of the skin samples revealed a chronic moderate to severe, septal to nodular, pyogranulomatous panniculitis with variable fibrosis and absence of vasculitis (Fig. 2A). Special stain included Gram and no etiological agents were visible. Flexible gastroduodenoscopy and colonoscopy were performed. The gastric body and pyloric antral mucosa surface exhibited moderate hyperemia. In addition, the pyloric antrum appeared moderately hemorrhagic and mildly edematous. Duodenal endoscopy examination revealed moderate friable and hyperemic mucosa with several areas of erosions. Colonoscopy yielded diffusely severe hyperemic friable mucosa with multifocal hemorrhages and ulcers. Gastric and colonic biopsies were taken simultaneously for histopathologic examination. A morphological diagnosis of chronic moderate to severe, necrotic to ulcerative, lymphoplasmacytic colitis and chronic mild to moderate, lymphocytic gastritis was made (Fig. 2B). 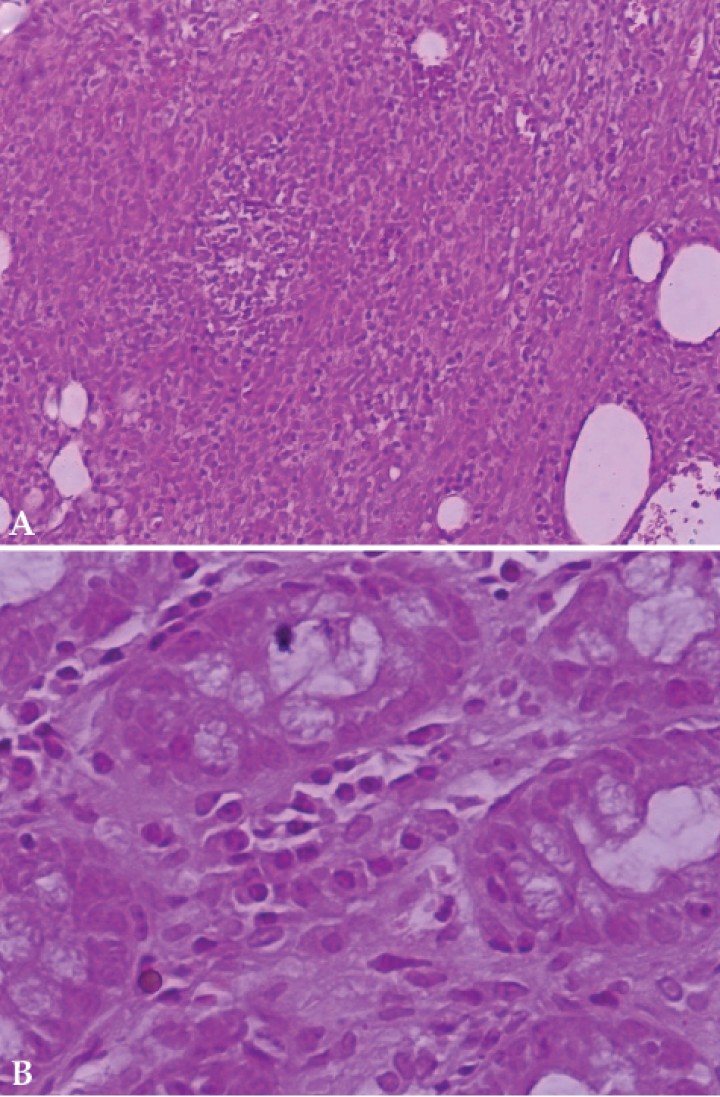
Figura 2
Histopathological evaluation of panniculitis and concurrent inflammatory bowel disease in a dog. (A) Photomicrograph. Nodular to septal, pyogranulomatous panniculitis (Haematoxylin and eosin x200). (B) Photomicrograph. Lymphoplasmacytic colitis. Excessive mononuclear infiltrate in lamina propria of mucosa (Haematoxylin and eosin x400).
Discussion
Histologically, inflammation in the subcutaneous adipose tissue is referred to as panniculitis.1,2 Clinically, the most common feature of panniculitis in humans and dogs is the presence of subcutaneous nodules, in humans most commonly distributed on lower extremities, while in dogs most frequently on the trunk and neck. In this dog, the main clinical finding consisted in multiple erythematous subcutaneous nodules, most of them localized over the trunk and neck. The clinical features observed were thus in accordance with previous findings.1,2,6 In humans, dogs and cats, multiple etiologic factors are involved in the genesis of panniculitis including infectious and noninfectious etiologies.1,2,6-10 In dogs and cats, infectious etiologies include bacterial, fungal, viral, protozoal and parasitic agents. While noninfectious etiologies include pancreatic disease, foreign body, immune-mediated, trauma, post-surgery, thermal burns, post-injection/vaccination reactions, vitamin E deficiency and idiopathic sterile nodular panniculitis (NP).1,6-10 In this case, no etiological agents were identified after histopathological evaluation, Gram stain, and aerobic and anaerobic bacterial, fungal and mycobacterial cultures from deep tissue. In addition, rt-PCR for Hepatozoon canis, Babesia spp, Anaplasma spp and Ehrlichia spp, and Leishmania infantum serology titer were also negative. No evidence of pancreatic disease was observed after abdominal ultrasonography and normal serum lipase, amylase and sTLI. However, an infectious etiology or pancreatic disease cannot be completely excluded. Other noninfectious causes such as foreign body, trauma, post-surgery, thermal burns, post-injection/vaccination reactions were ruled out after clinical history and histopathological evaluation. Currently there are no clear standardized specifications what minimum investigations need to be performed to define panniculitis as idiopathic in the dog. In this case, after thorough clinicopathological investigations an etiological agent was not identified. Hence, a diagnosis of panniculitis of unknown origin with concurrent IBD was established.
In human medicine, panniculitis is currently classified into predominantly septal and predominantly lobular forms, with or without vasculitis.2 Among the human panniculitides, EN is the most frequently-encountered clinicopathologic variant, and is considered the prototype of septal panniculitis without vasculitis.2,3 In the dog and cat, a similar classification has been adopted, and the predominantly lobular form is the most common histopathologic subtype observed.8 In this case, a septal to nodular panniculitis was observed. This pattern has been reported in two dogs in a recent retrospective study of canine idiopathic NP.6 In humans, the inflammatory infiltrate of EN varies with age of the lesion. In early lesions, edema, hemorrhage, and neutrophils are responsible for the septal thickening. Whereas in late-stage EN lesions, it may be difficult to establish whether the lesion is a mostly septal or mostly lobular panniculitis, because the entire subcutaneous tissue is effaced by a fibrotic and granulomatous process.3 This dog had a history of recurrent subcutaneous nodules. We cannot ascertain whether the fibrosis and pyogranulomatous inflammation observed at diagnosis could represent a late-stage lesion similar to EN or was present initially.
EN in humans is a cutaneous reactive process that has been reported in association with a wide variety of different diseases including infections, malignancies, drugs, autoimmune disorders and IBD. However, despite thorough clinical and laboratory investigations, the etiology of EN may remained unknown in up to 60% of the cases.2,3 In adults, EN associated with IBD often correlates with a flare-up of the disease, although the cutaneous eruption may precede the clinical appearance of the IBD.3,11 In human medicine, IBD refers to ulcerative colitis (UC) and Crohn’s disease (CD), two chronic idiopathic inflammatory diseases.4 It has been reported that ulcerative colitis UC is more frequently associated with EN than CD, in approximately 4% and 2% of patients respectively.3,11 In the dog, panniculitis remains a disease which may be associated with other illnesses.1,7 Well documented cases of EN-like lesions in dogs are scarce;12 however, it has been reported recently in two dogs associated with administration of potassium bromide.13 In the dog, IBD is the collective term for a group of chronic enteropathies characterized by persistent or recurrent gastrointestinal signs and inflammation of the GI tract.5 The underlying cause of IBD remains elusive. However, accumulating evidence suggests that intestinal inflammation results from altered interaction between gut microbiota and the mucosal immune system in a susceptible host. IBD is diagnosed after exclusion of extra-intestinal, infectious or parasitic diseases or inability to document other causes of gastrointestinal inflammation.5,14,15 In our case, concurrent IBD was diagnosed following the recommended diagnostic algorithm4,5 including fecal analysis, CBC, serum chemistry, urinalysis, sTLI, thoracic radiographs, abdominal ultrasound and gastrointestinal endoscopy examinations, and histopathological evaluation. The most common histopathologic lesion found is increased cellularity of the lamina propria. Increased numbers of lymphocytes and plasma cells is the most frequently reported in canine IBD, as it did occur in this case. Also, there is increasing evidence that chronic mucosal lymphoplasmacytic inflammation may be a precursor to the development of alimentary lymphoma in humans, dogs and cats. Thus, one of the greatest challenges for veterinary pathologists is sometimes differentiating between a chronic mononuclear inflammatory process and lymphoid neoplasia. To overcome this challenge immunophenotyping and clonality test have been recommended. As neither of these tests was performed in this case we cannot completely exclude a neoplastic infiltrate. However, this distinction is a major issue in feline medicine and secondly, follow-up and clinical response favors inflammatory process. A surface epithelial injury is one of the morphologic criteria in the histopathological examination of the colonic mucosa and ranges from normal to marked with ulceration, the latter was found in our case.5,14,15
Thus far, idiopathic panniculitis and concurrent IBD has been suspected but not confirmed in two dogs.9,10 In one dog, the retrospective nature of the study resulted in inconsistency in testing for infectious agents. However, the exact diagnostic workup for that dog was not reported and it remains possible that a comprehensive approach to rule out infectious diseases were performed.9 In the other dog, concurrent IBD was suspected, but endoscopic and histopathologic examinations of the gastrointestinal tract and bacterial and mycobacterial cultures were not performed.10 Endoscopy of the gastrointestinal tract is recommended for diagnosis of IBD and histopathologic evaluation of biopsy specimens is required for definitive diagnosis of IBD.14 Both endoscopy and histopathology were performed in this dog and results were consistent with IBD. Furthermore, IBD dietary management with a hydrolyzed diet had prevented relapses of the nodules after discontinuation of medical treatment, three months after diagnosis, and after 12 months. In a recent review it has been reported that 50% of dogs diagnosed with IBD responded to a hydrolyzed diet.15 In human medicine, it has been reported that control of IBD may prevent further EN.16 However, we cannot completely exclude spontaneous remission of the skin nodules.6,7 In addition, it remains unclear the true association between the paniculitis and the IBD. That is because IBD is very common, Dachshunds appear to be predisposed to sterile nodular paniculitis and IBD and concurrent paniculitis is very rarely reported.1,9,10
In conclusion, this case report describes a dog with panniculitis of unknown etiology, after a comprehensive testing including bacterial, mycobacterial and fungal cultures, and concurrent IBD. Endoscopic and histological examinations confirmed IBD. After 12 months, long-term dietary treatment for IBD prevented relapses of the skin nodules. Similar findings have been reported in human medicine. In dogs with panniculitis, in which no underlying cause can be identified, and exhibiting gastrointestinal signs, IBD should be excluded after endoscopic and histopathological examinations.
Sources of funding: this study was self-funded.
Conflict of interest: no conflicts of interest have been declared.
Bibliografía
- 1.
Miller WH Jr, Griffin DE, Campbell KL. Miscellaneous skin diseases. In Miller WH Jr, Griffin DE, Campbell KL (ed): Muller and Kirk’s Small Animal Dermatology, St. Louis, Elsevier Health 2013; 701-704.
- 2.
Aronson IK, Fishman PM, Worobec SM. Panniculitis. In Lowell A. Goldsmith, Stephen I. Katz, Barbara A. Gilchrest, Amy S. Paller, David J. Leffell, Klaus Wolff (ed): Fitzpatrick’s Dermatology in General Practice, New York, Mc Graw Hill, 2012; 732-755.
- 3.
Requena L, Yus ES. Erythema nodosum. Dermatol Clin 2008; 26: 425-438.
[PubMed] - 4.
Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care 2017; 44: 673-692.
[PubMed] - 5.
Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract 2011; 41: 381-398.
[PubMed] - 6.
Contreary CL, Outerbridge CA, Affolter VK, et al. Canine sterile nodular panniculitis: a retrospective study of 39 dogs. Vet Dermatol 2015; 26: 451-e105.
[PubMed] - 7.
Yamagishi C, Momoi Y, Kobayashi T et al. A retrospective study and gene analysis of canine sterile panniculitis. J Vet Med Sci 2007; 69: 915-924.
[PubMed] - 8.
Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Diseases of the panniculus. In Thelma Lee Gross, Peter J. Ihrke, DEmily J. Walder, Verena K. Affolter (ed): Skin Diseases of the Dog and Cat. Clinical and Histopathologic Diagnosis, Ames, Blackwell Science Ltd, 2005; 538-558.
- 9.
O’Kell AL, Inteeworn N, Diaz SF, et al. Canine sterile nodular panniculitis: a retrospective study of 14 cases. J Vet Intern Med 2010; 4: 278-284.
[PubMed] - 10.
Dandrieux JR, Timm K, Roosje PJ, et al. Unusual systemic signs in a dog with sterile neutrophilic-macrophagic lymphadenitis and nodular panniculitis. J Am Anim Hosp Assoc 2011; 47: 117-121.
[PubMed] - 11.
Paller AS. Cutaneous changes associated with inflammatory bowel disease. Pediatr Dermatol 1986; 3: 439-445.
- 12.
Yager JA, Wilcock BP. Panniculitis. En Julie A. Yager, Brian P. Wilcock (ed). Color atlas and text of surgical pathology of the dog and cat: Dermatopathology and skin tumors, London, 1994; 199-214.
- 13.
Boynosky NA, Stokking LB. Potassium bromide-associated panniculitis. J Small Anim Pract 2014; 55: 640-642.
[PubMed] - 14.
Washabau RJ, Day MJ, Willard MD et al. WSAVA International Gastrointestinal Standardization Group. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010; 24:10-26.
[PubMed] - 15.
Dandrieux JRS. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same?. J Small Anim Pract 2016; 57: 589-599.
[PubMed] - 16.
Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician 2007; 75: 695-700.
[PubMed]



