Diaphragmatic plication in a dog with bilateral paralysis and muscle degeneration
Plicatura diafragmática en un perro con parálisis bilateral y degeneración muscular
G. Santarelli, J.D. Carrillo, A. Bernabé, M.A. Gómez-Sánchez, A. Agut, M. J. Fernández-del Palacio
Contacto: Giorgia.Santarelli@ed.ac.uk
Resumen
Un perro de raza Bulldog Francés, hembra entera, acudió al hospital por un cuadro de intolerancia al ejercicio y dificultad respiratoria que aparecía al realizar esfuerzo desde hacía 3 semanas. Al examen físico se detectaron taquipnea y respiración paradójica exacerbadas por el estrés de la manipulación, y un abdomen anormalmente excavado. Las radiografías torácicas mostraron desplazamiento craneal del diafragma y campos pulmonares reducidos. En base a los hallazgos clínicos y radiográficos se sospechó una disfunción diafragmática y con fluoroscopia y ecografía en modo M se diagnosticó una parálisis diafragmática bilateral. Un mes después del diagnóstico y debido a la ausencia de mejoría clínica con tratamiento conservador, se realizó una plicatura quirúrgica del diafragma; el procedimiento se consideró exitoso, y resultó en una remisión duradera de los signos clínicos. El estudio histopatólogico de una muestra obtenida durante el procedimiento quirúrgico reveló degeneración muscular y necrosis. Este es el primer caso descrito de un perro con parálisis diafragmática bilateral y degeneración muscular identificada por histopatología, y con una resolución duradera del cuadro clínico después de la plicatura quirúrgica.
Palabras clave: parálisis diafragmática, plicatura diafragmática, respiración paradójica, perro.
Clin. Vet. Peq. Anim, 2017, 37 (4): 257 - 262
Summary
A one-year-old intact female French Bulldog was evaluated with a 3-week history of exercise intolerance and respiratory distress on exertion. An abnormally slim abdomen, tachypnea and marked paradoxical abdominal respiration exacerbated by stress were noted during clinical examination. Thoracic radiographs showed bilateral cranial displacement of the diaphragm and small lung fields. Diaphragmatic dysfunction was suspected based on clinical and radiographic findings, and bilateral diaphragmatic paralysis was diagnosed through fluoroscopy and M-mode ultrasonography. Surgical plication of the diaphragm was performed one month after diagnosis because no clinical improvement was achieved with conservative management; the procedure was considered successful, and resulted in a prompt and long-lasting remission of clinical signs. Histopathology of a biopsy specimen of the muscle obtained during surgery revealed the presence of myofiber degeneration and necrosis. This is the first reported case of bilateral diaphragmatic paralysis and muscle degeneration identified by histopathology in a dog, with a favorable long-term outcome following surgical plication via laparotomy.
Keywords: diaphragmatic paralysis, diaphragmatic plication, paradoxical respiration, dog.
Clin. Vet. Peq. Anim, 2017, 37 (4): 257 - 262

La presencia de este logo en un artículo de la revista indica que se publicará un examen sobre el mismo en la plataforma AVEPA Elearning. Su resolución aporta 0,15 créditos dentro del sistema de acreditaciones de especialidades veterinarias de AVEPA.
Introduction
Diaphragmatic paralysis is an uncommonly reported condition in animals, described in a few dogs and cats, llamas and a pony.1-6 In humans, the condition is often of unknown origin, however it can represent the result of central or peripheral neurologic injuries or degeneration, neuromuscular junction diseases or diaphragmatic myopathies.6-10 Severe bilateral dysfunction of the diaphragm can cause respiratory distress, and a paradoxical abdominal respiratory pattern due to the passive cranial movement of the muscle in response to the negative inspiratory pressure.6 However, these signs are nonspecific, and diaphragmatic paralysis can be challenging to diagnose. Associated radiographic signs such as cranial displacement of the diaphragm or absence of muscle movement comparing inspiratory and expiratory views can be overlooked especially with bilateral involvement.1,5,10 Nevertheless, functional imaging including fluoroscopy and ultrasonography can be very useful and relatively easy to perform, while electromyography, respiratory function tests and transthoracic test studies, although of value, are less commonly employed in the clinical setting.1,2,4,6 Surgical treatment can be necessary in some patients with this condition, and successful diaphragmatic plication has been previously described in a dog.2 This clinical case reports on the signalment, clinical signs, diagnostic results including histopathology, and long-term outcome in a young dog with diaphragmatic paralysis surgically corrected via plication of the muscle.
Clinical report
A one-year-old intact female French Bulldog was referred to the Cardiopulmonary Service of the Veterinary Teaching Hospital of the University of Murcia because of a 3-week history of exercise intolerance and respiratory distress on exertion. The dog was almost asymptomatic at rest, and had recovered from an uncomplicated gastroenteritis 3-4 days before the acute onset of symptoms. Previous treatments for current condition included repeated dexamethasone injections (unknown dosage, SC) administered by the referring veterinarian, benazepril (0.5 mg/Kg q24 h, PO; Fortekor, Elanco, Huningue, FR) and furosemide (4 mg/Kg q24 h, PO; Seguril, Sanofi-Aventis, Barcelona). The owner had dispensed the latter two medications during the previous 15 days without observing any clinical improvement. The animal had maintained normal eating and drinking habits during this time. The owners did not report weight loss or abnormal feces; deworming and vaccination were up-to-date, and there was no travel history.
On physical examination, the dog was bright, alert and responsive, with a body condition score of 5/9, and an abnormally slim abdomen (Fig. 1). The posture was slightly orthopneic, and the breathing pattern was characterized by marked paradoxical abdominal respiration, with an increased respiratory rate of 56 rpm. Tachypnea and respiratory distress were exacerbated by stress during clinical examination, and auscultation of the cranial lung fields revealed increased vesicular breath sounds bilaterally. The remaining physical examination findings were unremarkable. Test results of CBC and serum biochemistry revealed mild lymphopenia (lymphocytes count, 910 cells/µL; reference interval, 1,000 to 5,800 cells/µL) and mild hyperglucemia (113 mg/dL; reference interval, 60 to 100 mg/dL).
Figura 1
An abnormally slim abdomen is evident in this French Bulldog with bilateral diaphragmatic paralysis.
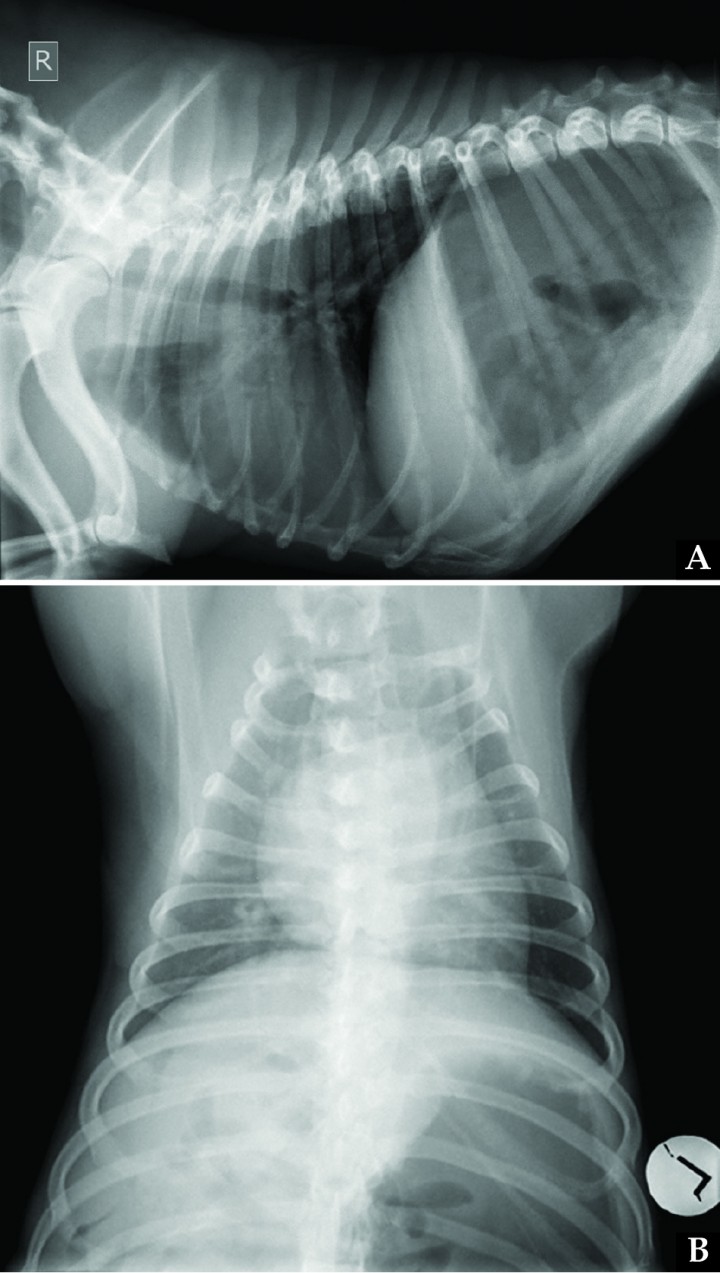
Pleural space diseases, diaphragmatic disorders, and pulmonary parenchymal diseases were assessed most likely based on the paradoxical abdominal respiratory pattern and the absence of noisy breathing. Thoracic radiographs (Fig. 2) showed bilateral cranial displacement of the diaphragm and small lung field. On the left lateral view, the left crus intersected the spine at the cranial border of T9, while on the right lateral view, the right crus intersected the spine at the cranial border of T10; on the dorsoventral view, the most cranial edge of the diaphragm was visible at the level of the cranial border of T8. Diffuse increased opacity of the entire lung field was observed, consistent with poor lung inflation. Cardiovascular structures appeared normal. According to the vertebral heart score system, cardiac silhouette measured 11.6 vertebral units including thoracic hemivertebrae (reference interval in Bulldogs with abnormal vertebrae, 11.8 to 15 vertebral units).11 Severe gastric distension with gas was also observed in the cranial abdomen.
Figura 2
(A) Right lateral and (B) dorsoventral thoracic radiographs of the same dog in Figure 1 on admission. Due to bilateral diaphragmatic paralysis, cranial diaphragmatic displacement is present, with subsequent poor lung inflation and increased opacity of the lung field. Gastric dilation is also visualized.
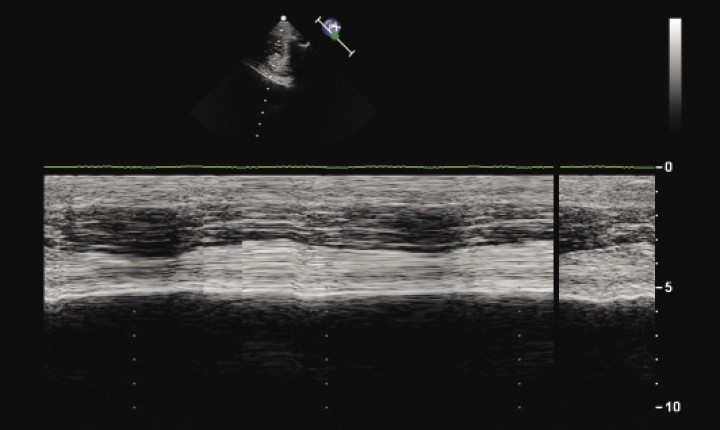
Considering the radiological findings, differential diagnoses at this point included dysfunction of the diaphragm (including paralysis and weakness), bilateral pleural adhesions, recurrent gastric dilation and, less likely, radiographs taken in expiration. Functional imaging with fluoroscopy, B-mode and M-mode ultrasonography of the diaphragm was planned to further evaluate diaphragmatic motion. Fluoroscopy revealed absence of diaphragm excursion during inspiration, and enhanced rib movement. Absence of movement of diaphragmatic crura was also detected upon M-mode ultrasonography (Fig. 3). These findings were consistent with bilateral diaphragmatic paralysis.
Figura 3
M-mode ultrasonography image of the left hemidiaphragm for the dog in Figure 1 shows absence of diaphragmatic movement.
Final diagnosis was bilateral diaphragmatic paralysis of unknown origin. Treatment for heart disease was discontinued, and conservative treatment with prednisone (1 mg/Kg [0.45 mg/lb], PO, q 24 h; Dacortin, Merck, S.L., Mollet del Vallés, Barcelona) and exercise restriction was prescribed.
Remission of clinical signs was not achieved after one month, and surgical treatment was recommended. Open transabdominal surgical plication of the diaphragm was planned to improve the function of the paralyzed diaphragmatic musculature. After median celiotomy, the cranial abdomen was explored, and the diaphragm appeared slightly discoloured, flaccid and lax. Both the left and right leaflets of the diaphragmatic central tendon were plicated using interrupted, inverting polypropylene sutures in an interlocking pattern, resulting in a tense, non-mobile diaphragm.
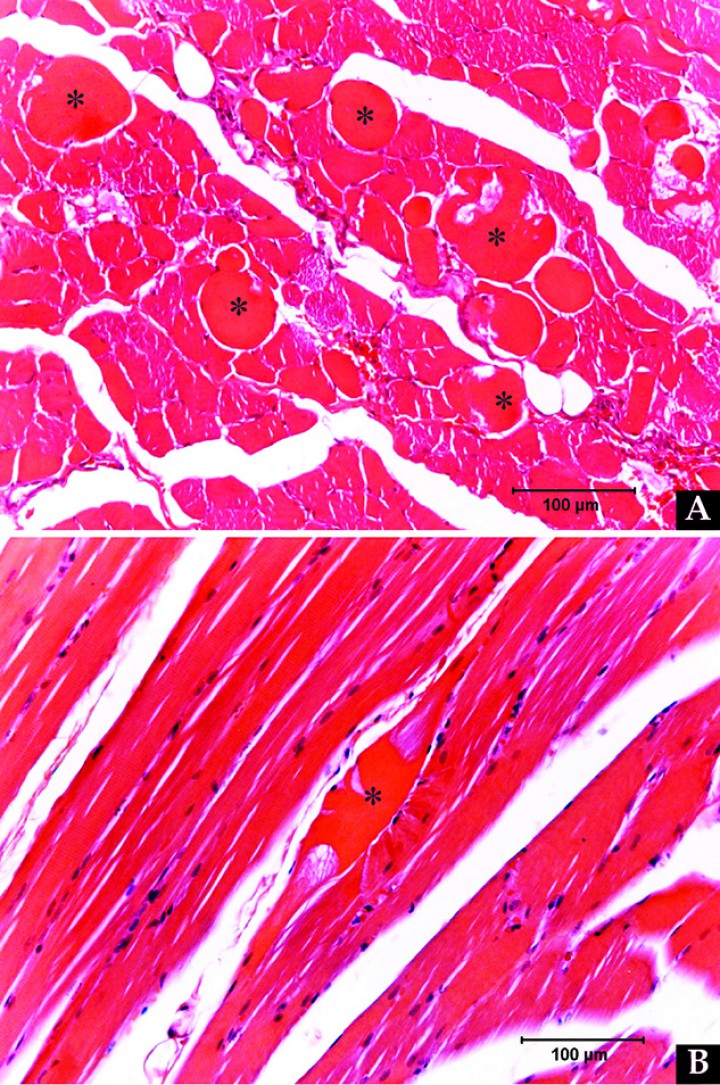
Biopsy specimens of the diaphragm musculature were obtained and submitted for histopathological examination. Changes observed in the diaphragmatic muscle included multifocal segmental myofiber degeneration/necrosis, characterized by myofiber swelling, hypereosinofilia, loss of cross striations and lack of normal cylindrical morphology (Fig. 4). Mild myositis was also present, characterized by minimal eosinophilic perivascular infiltration. Histopathological diagnosis was mild to moderate idiopathic myofiber degeneration and necrosis.
Figura 4
Biopsy specimens of the diaphragmatic musculature show mild to moderate multifocal segmental myofiber degeneration/necrosis. Changes observed in the abnormal myofibers (*) visualized in (A) transversal section and (B) longitudinal section include myofiber swelling, loss of cross striations and lack of normal cylindrical morphology.
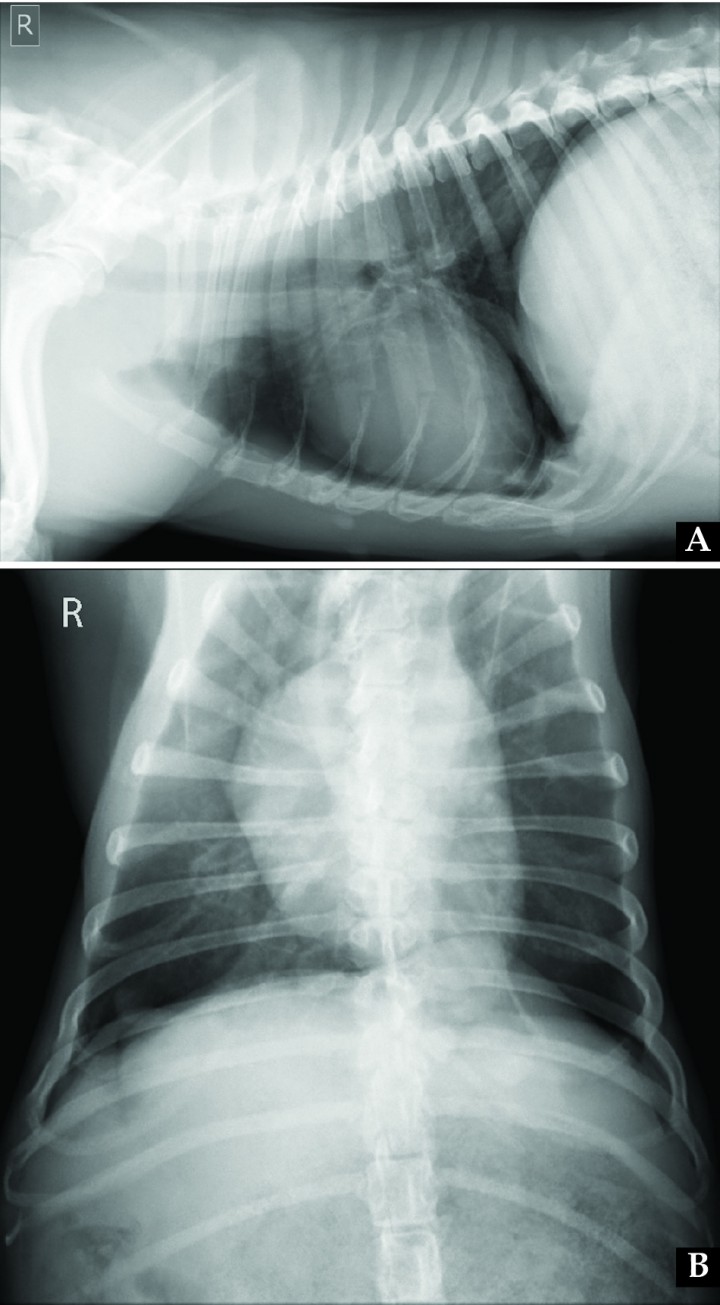
The dog recovered uneventfully from surgery, and immediately showed marked improvement of clinical signs, so that two days after was discharged from the hospital with no medical treatment. Exercise restriction and weight control were recommended. At 1-month revision, the patient presented in a good clinical condition, with a normal breathing pattern and abdominal morphology (Fig. 5), and the owner reported only mild dyspnea during exercise. Control radiographs were obtained (Fig. 6). On the dorsoventral view, normal position of the right diaphragmatic crus was observed, while on the left side cranial displacement of the left crus was present, so that partial suture loosening was suspected and close monitoring of evolution was advised. Six months after surgery the owner referred by telephone no clinical signs at rest or with mild or moderate physical activity, while strenuous exercise was still not allowed. Eighteen months after surgery, the dog was clinically normal according to the owner and referring veterinarian, and the recommended rechecks were therefore missed, also due to travel issues.
Figura 5
After diaphragmatic plication, the abdomen shows a normal shape.
Figura 6
(A) Right lateral and (B) dorsoventral thoracic radiographs obtained one month after surgical diaphragmatic plication. On the lateral view, diaphragm position appeared more normal when compared to Figure 2. On the dorsoventral view, normal position of the right diaphragmatic crus is observed, while on the left side cranial displacement of the left crus is present, which might be related to partial suture loosening.
Discussion
This is the first reported case of bilateral diaphragmatic paralysis and muscle degeneration identified by histopathology in a dog, with a favorable outcome following surgical plication via laparotomy. In previously reported canine spontaneous cases, final diagnoses were post-surgical unilateral diaphragmatic paralysis in a young adult German Shepherd, suspected bilateral phrenic neuritis in a Golden Retriever puppy, bilateral diaphragmatic paralysis of unknown origin in a 13-year-old Yorkshire Terrier, and unilateral diaphragmatic paralysis of unknown origin in a young adult Maltese dog.1-3 In cats, two cases of transient post-traumatic hemidiaphragmatic paralysis have been reported, and bilateral palsy has been described in a cat with Toxoplasma gondii polymyositis.4 Further cases of either unilateral or bilateral diaphragmatic paralysis were reported in another dog, a llama, a pony, and 11 alpacas.4-6 In the latter species, when necropsy was performed, degenerative changes were identified in various nerves, including the phrenic ones, sometimes accompanied by myofiber atrophy and myositis of the diaphragmatic muscle, and in the absence of detectable etiologic agents.6
In humans, the etiology remains unclear in more than two-third of the cases. The remainder of patients most frequently experience diaphragmatic paralysis as a consequence of thoracic surgery, due to traction of the phrenic nerve applied during the procedure. Other identified causative conditions that provoke stretching, compression or destruction of the phrenic nerves include traumatic injuries or blunt traumas to the chest or neck, birth traumas, inadequate cervical manipulations and intrathoracic malignancies. Further, bilateral diaphragmatic paralysis has been reported in neurodegenerative and neuromuscular disorders, accompanying a generalized polyneuropathy or being the initial manifestation of a multifocal motor neuropathy. Eventually, postviral neuropathies with phrenic nerve involvement have also been described as sequelae of infections like poliovirus, herpes zoster virus, West Nile virus, and HIV infections. Idiopathic neuritis is suspected when a cause cannot be identified.7-10
In the absence of detectable neurologic deficits or predisposing mechanisms of injury such as high spinal cord injuries, bilateral diaphragmatic paralysis may represent the result of a disease of both peripheral phrenic nerves, of the neuromuscular junctions of the diaphragm or of the diaphragmatic muscle itself.2 In the case reported here, muscle degeneration and necrosis were identified through histopathology, and a diaphragmatic myopathy was suspected; though, as a laparotomic approach was employed for diaphragmatic plication, no biopsy specimens of the phrenic nerve were obtained, and concurrent lesions could not be excluded. Myofiber degeneration and necrosis might have been a consequence of injury due to chemical irritants/myotoxins, trauma, or inflammation/infection.12 Due to the previous history of a recent gastroenteritis, a common infectious origin was suspected, but could not be confirmed.
Diaphragmatic dysfunction is quite challenging to diagnose, and can be classified as paralysis, weakness or eventration.10 In the presence of diaphragmatic paralysis or pronounced weakness, the negative pressure created at inspiration by the other respiratory muscles causes a passive cranial movement of the diaphragm, as opposed to its normal active caudal displacement. Methods used to evaluate the suspected dysfunctional diaphragm focus therefore on observing the direction of movement, or measuring the pressure in the thoracic cavity.13
One should suspect dysfunction of the diaphragm (paralysis and weakness) in the differential diagnosis of a cranially displaced diaphragm at chest radiography, or when no position differences are detected comparing inspiratory and expiratory views.1,5,10 The dorsal portion of the diaphragmatic crura attaches to the ventral surfaces of the 3rd and 4th lumbar vertebrae, and on lateral survey radiographs, the intersection point of the diaphragm and spine appears normally located between T11 and T13, although it may vary between T9 and L1.1,5,14 However, radiographic findings can be easily misinterpreted, especially when bilateral displacement exists and the normal shape of the diaphragm is maintained.
In veterinary medicine, other diagnostic tools used in reported clinical cases included fluoroscopy, B-mode ultrasonography, M-mode ultrasonography, and electromyography, while respiratory function tests and transthoracic test studies are not commonly employed in the clinical setting.1,2,4,6 Functional imaging with fluoroscopy or ultrasonography is simple to perform, and effective in diagnosing diaphragmatic dysfunction. At fluoroscopic examination, diaphragmatic paralysis is indicated by the absence of diaphragmatic excursion on quiet and deep breathing, while diaphragmatic weakness is indicated by reduced or delayed excursion on deep breathing.10 Fluoroscopic images may be difficult to interpret, especially if bilateral paradoxical motion is present due to vigorous chest wall motion; this symmetric motion may at first appear normal, and it has to be recognized that the hemidiaphragms are in fact passively following the ribs on inspiration.5,7,10 In humans, a fluoroscopic sniff test is used to aid diagnostics, and if paradoxical motion of the diaphragm is visualized, paralysis or pronounced weakness is confirmed.10 M-mode ultrasonography has also been described in dogs and people as a valuable tool to assess diaphragmatic motion, representing a relatively simple and objective technique, which spares radiation hazards for the examiner and assisting persons compared to other imaging technologies such as fluoroscopy.3,13 In the case reported here, fluoroscopy and M-mode ultrasonography were of great value to confirm the diagnosis of diaphragmatic paralysis.
Specific criteria to decide whether surgical resolution is necessary are lacking in veterinary medicine. A proposed criterion in adult humans is that a significant improvement in clinical status and arterial blood gases is achieved when the patient is mechanically ventilated with continuous positive airway pressure, indicating that the diaphragmatic paralysis is the main cause of respiratory distress. Furthermore, it is recommended to delay surgery in these patients, as an example for a prudent period of 4 to 6 weeks, as the condition could be transient.15 In the dog of our report, arterial blood gases were not determined to avoid stress during blood sample obtainment, and mechanical ventilation was not considered necessary based on clinical status. Surgery was eventually recommended as no clinical improvement was achieved with conservative management one month after the diagnosis, and a spontaneous resolution was not deemed likely; seeing the favorable clinical evolution, diaphragmatic plication is considered to have been beneficial in this dog, at least in the short-term. In fact, we cannot completely exclude a later spontaneous recovery of the diaphragmatic function that could have influenced the long-term favorable outcome, as the patient was unfortunately not available for a proper follow-up at the hospital.
In conclusion, in dogs with respiratory distress, a paradoxical abdominal respiratory pattern and a cranially displaced diaphragm on chest X-rays should raise the suspicion of diaphragmatic dysfunction (paralysis or weakness). Other diagnostic tools such as fluoroscopy and M-mode ultrasonography are recommended to confirm the diagnosis and monitor evolution. If permitted by the clinical status, an initial prudent period of conservative treatment could be indicated, and when there is no evidence of spontaneous recovery of muscular function, surgical plication can represent a beneficial procedure to alleviate symptoms.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article.
Bibliografía
- 1.
Suter PF, Lord PF. Abnormalities of the diaphragm. In: Suter PF, ed. Thoracic Radiography. A text atlas of thoracic diseases of the dog and the cat. Wettswill: Suter, 1984; 179-204.
- 2.
Greene CE, Basinger RR, Whitfield JB. Surgical management of bilateral diaphragmatic paralysis in a dog. J Am Vet Med Assoc 1988; 193:1542-1544.
[PubMed] - 3.
Choi M, Lee N, Kim A, et al. Evaluation of diaphragmatic motion in normal and diaphragmatic paralyzaed dogs using M-mode ultrasonography. Vet Radiol Ultrasound 2014; 55:102-108.
- 4.
Vignoli M, Toniato M, Rossi F, et al. Transient post-traumatic hemidiaphragmatic paralysis in two cats. J Small Anim Pract 2002; 43:312-316.
- 5.
Park RD. The diaphragm. In: Thrall DE, ed. Textbook of veterinary diagnostic radiology. 5th ed. St. Louis: Saunders Elsevier, 2007; 525-540.
- 6.
Byers S, Barrington G, Nelson D, Haldorson G, Holt T, Callan R. Neurological causes of diaphragmatic paralysis in 11 alpacas (Vicugna pacos). J Vet Intern Med 2011; 25:380-385.
[PubMed] - 7.
Liet JM, Dejode JM, Joram N, et al. Bedside diagnosis of bilateral diaphragmatic paralysis. Intensive Care Med 2013; 39: 335.
- 8.
Melero MJ, Mazzei ME, Bergroth B, Cantardo DM, Duarte JM, Corti M. Bilateral diaphragmatic paralysis in an HIV patient: Second reported case and literature review. Lung India 2014; 31:149-151.
- 9.
Schram DJ, Vosik W, Cantral D. Diaphragmatic paralysis following cervical chiropractic manipulation. Chest 2001; 119:638-639.
[PubMed] - 10.
Nason LK, Walker CM, McNeeley MF, Burivong W, Fligner CL, Godwin JD. Imaging of the diaphragm: anatomy and function. Radiographics 2012; 32(2):E51-E71.
[PubMed] - 11.
Jepsen-Grant K, Pollard RE, Johnson LR. Vertebral heart scores in eight dog breeds. Vet Radiol & Ultrasound 2013; 54:3-8.
[PubMed] - 12.
Crabbs TA. Skeletal muscle – degeneration. In: Cesta MF, Herbert RA, Brix A, et al, eds. National Toxicology Program Nonneoplastic Lesion Atlas; 17 August, 2015.
[NTP] - 13.
Lloyd T, Tang Y-M, Benson MD, King S. Diaphragmatic paralysis: the use of M mode ultrasound for diagnosis in adults. Spinal cord 2006; 44:505-508.
[PubMed] - 14.
Llabrés-Díaz F, Petite A, Saunders J, Schwarz T. The thoracic boundaries. In: Schwarz T, Johnson V, eds. BSAVA Manual of canine and Feline Thoracic Imaging. Quedgeley, UK: BSAVA, 2008: 340-376.
- 15.
Abad P, Lloret J, Martínez Ibañez V, Patiño B, Boix-Ochoa J. Parálisis diafragmática: patología al alcance del cirujano pediátrico. Cir Pediatr 2001; 14:21-24.
[PubMed]